 | |
| Names | |
|---|---|
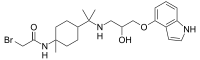
| IUPAC name
2-Bromo-N-[4-(2-{[2-hydroxy-3-(1H-indol-4-yloxy)propyl]amino}-2-propanyl)-1-methylcyclohexyl]acetamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H34BrN3O3 | |
| Molar mass | 480.447 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Pindobind is a compound developed by researchers associated with Stanford University,[1] identified as a central nervous system depressant,[2] which generated a response in animals reducing offensive actions such as chasing, while also notably reducing tendencies of the test animal to evade when stimulated to do so.[2] It acts as an irreversible beta blocker and irreversible 5-HT1A receptor antagonist.
See also
References
- ↑ Peroutka, Stephen J.; Pitha, Josef (Jul 20, 1993), Method for relieving anxiety using 5-hydroxytryptamine-1a-receptor-binding compounds, retrieved 2016-06-04
- 1 2 Bell, R; Hobson, H (1993). "Effects of pindobind 5-hydroxytryptamine1A (5-HT1A), a novel and potent 5-HT1A antagonist, on social and agonistic behaviour in male albino mice". Pharmacology Biochemistry and Behavior. 46 (1): 67–72. doi:10.1016/0091-3057(93)90318-N. PMID 8255924.
| β, non-selective | |
|---|---|
| β1-selective | |
| β2-selective | |
| α1- + β-selective | |
| |
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.